- cross-posted to:
- [email protected]
- [email protected]
- cross-posted to:
- [email protected]
- [email protected]
Aging can seem like an unregulated process: As time marches along, our cells and bodies inevitably accumulate dings and dents that cause dysfunctions, failures and ultimately death. However, in 1993 a discovery upended that interpretation of events. Researchers found a mutation in a single gene that doubled a worm’s life span; subsequent work showed that related genes, all involved in the response to insulin, are key regulators of aging in a host of animals, from worms and flies to humans. The discovery suggested that aging is not a random process — indeed, specific genes regulate it — and opened the door to further research into how aging proceeds at a molecular level.
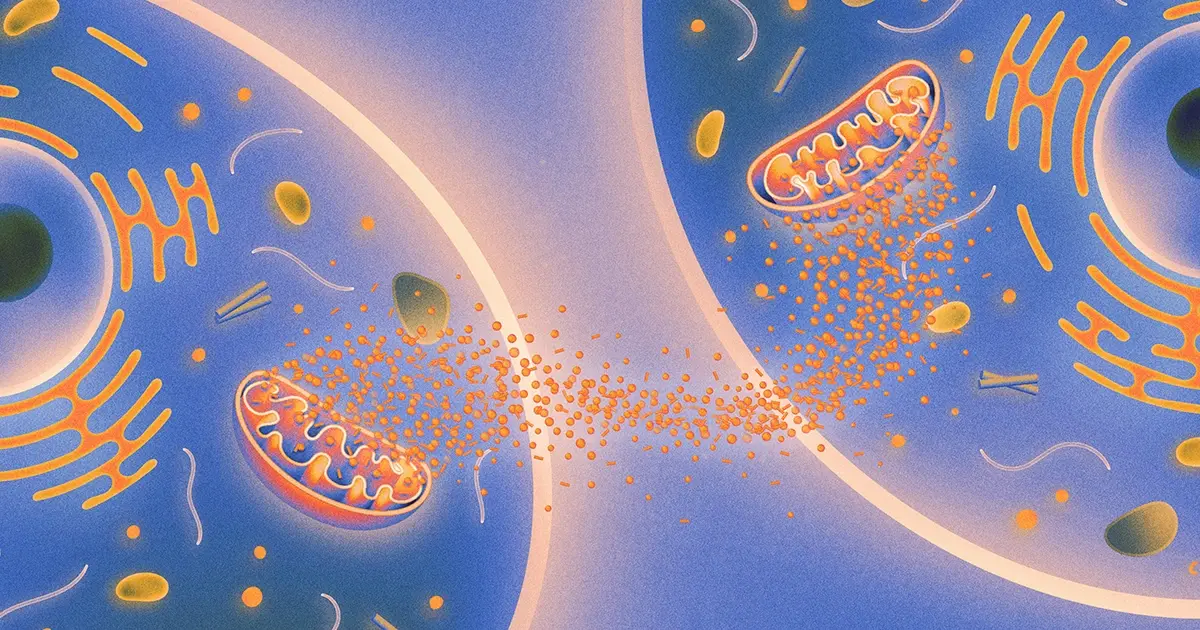
Recently, a set of papers documented a new biochemical pathway that regulates aging, one based on signals passed between mitochondria, the organelles best known as the powerhouse of the cell. Working with worms, the researchers found that damage to mitochondria in brain cells triggered a repair response that was then amplified, setting off similar reactions in mitochondria throughout the worm’s body. The effect of this repair activity was to extend the organism’s life span: The worms with repaired mitochondrial damage lived 50% longer.
What’s more, cells in the germline — the cells that produce eggs and sperm — were central to this anti-aging communication system. It’s a finding that adds new dimensions to the fertility concerns implied when people talk about aging and their “biological clock.” Some of the findings were reported in Science Advances and others were posted on the scientific preprint server biorxiv.org in the fall.


I’m 40. Can I have some more of that time too?
I’m a millennial, I’ve seen enough, ty.
But please don’t let capitalism force me to be alive longer just so I can work (ultimately meaningless tasks) longer.