I have come to think of it as all being probability fields.
When studying a particle, one cannot know both the energy and position of that particle with certainty (Heisenberg’s Uncertainty Principle). When chemists think about the 3d “structure” of atoms and molecules, they represent the nucleus as a tiny little ball and the electrons as bubbles of probability: .
The nucleus itself is in constant motion as well, and compared to the size of the actual protons and neutrons, there is much more empty space - kind of like planets in a solar system. And each of these protons/neutrons is composed of tiny particles called quarks, which again are in constant motion and thus make up probability fields that we call protons and neutrons.
I have come to think of it as all being probability fields.
When studying a particle, one cannot know both the energy and position of that particle with certainty (Heisenberg’s Uncertainty Principle). When chemists think about the 3d “structure” of atoms and molecules, they represent the nucleus as a tiny little ball and the electrons as bubbles of probability: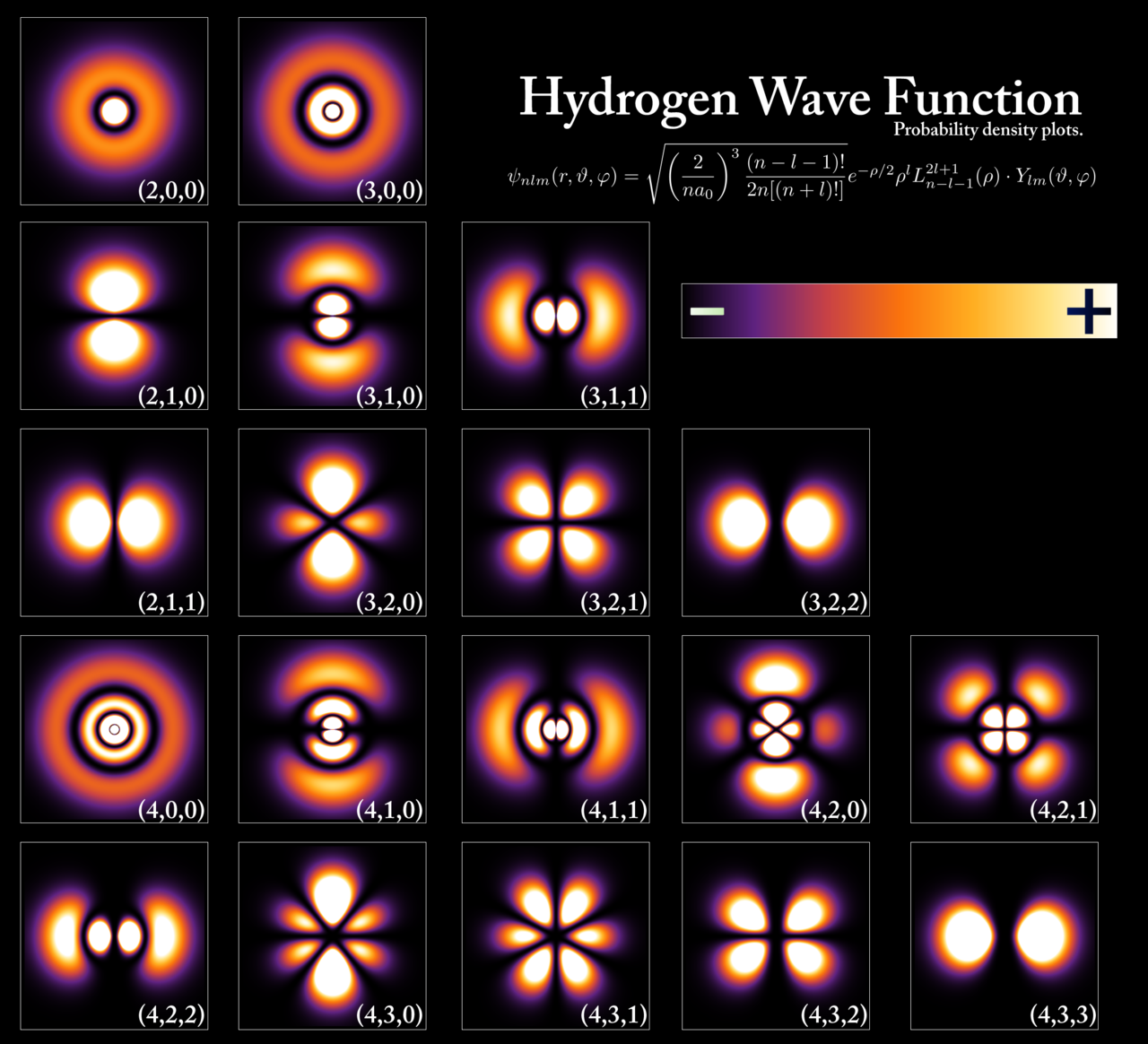 .
.
The nucleus itself is in constant motion as well, and compared to the size of the actual protons and neutrons, there is much more empty space - kind of like planets in a solar system. And each of these protons/neutrons is composed of tiny particles called quarks, which again are in constant motion and thus make up probability fields that we call protons and neutrons.