A team of researchers in Kansai is set to begin clinical trials next month to develop medicine to help grow teeth.
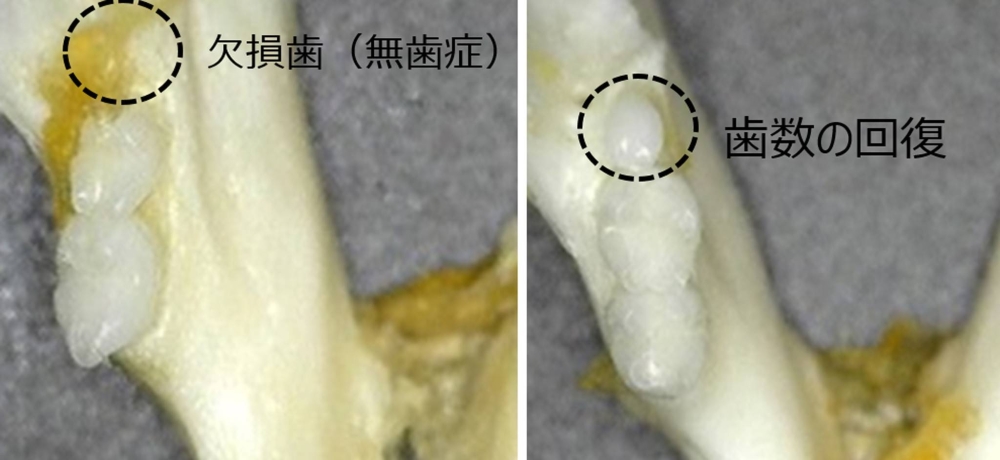
The researchers, including from Kitano Hospital and Kyoto University Hospital, will conduct trials for teething medicine, aimed at treating people with congenital anodontia who are born with few teeth.
To check its safety, the experimental drug will first be administered to adult men who have lost back teeth, before it is tested on children with congenital anodontia. The team aims to put the treatment into practical use in around 2030.


One of the most exciting developments in recent times
Not actually. This doesn’t do anything for most normal people. It’s for people who didn’t properly have a normal 2nd set of teeth. So if you just had your “adult” set of teeth go bad, this won’t help you.
*After doing a little more research, my above statement is correct. This drug only inhibits the protein that people with congenital anodontia have, so those people are able to start growing teeth. Less than 1% of people have congenital anodontia.
The little mention in the article about it leading to growing teeth in people who have lost them due to cavities (as the person with all the upvotes that replied to me here) is complete hyperbole in the article. This protein suppression medication can’t work on someone with a normal protein. It was just mentioned as fluff to get more press about “future possibilities”.
The article says that they hope to regrow teeth for people who have lost their teeth to cavities, and the initial test is being done on adults who have lost back teeth. So pretty much the exact target audience you say it wouldn’t target.
It’s really weird though. Why specify that you’re targeting specifically kids with a disorder when your treatment is being tested on adults, and would work on any adult who has lost teeth for any reason?
Because /hobthrob is incorrect. This medication will not and cannot be used to regrow teeth in people that don’t have the genetic disorder “congenital anodontia”. The drug can only help that >1% with the disorder.
That’s not what the article says though
Speculating, but probably because kids without any teeth and a genetic basis of that disease would pay for this treatment out of medical coverage, not dental coverage, at least in the US, and getting it approved for specifically that indication is easier, faster, and likely higher profit than the admittedly larger population but smaller insured availability of funds that would be the dental market.
Markets shouldn’t drive drug research, public health benefit should, but shrugs
Good thought, but it looks like they’re a Japanese team conducting trials in Japan, so the US excuse for an insurance system shouldn’t be a factor? IDK
Quick search tells me that they have a national dental plan that covers 70%, but has limits on which procedures materials etc are covered. In other words, they may not cover this procedure if it’s more expensive than a traditional implant.
Additionally, there’s harmonization efforts between Japan and the US FDA; if fully expect they’re running clinical trials in JP with hopes for a later release abroad, and the US is a huge market so they’d still be angling that I’d think.
My bad. I guess this is a completely different thing from one I read about around 6 months ago, then.
*Nevermind. This is the same thing and everyone else in here is mistaken. Go read any other articles about this. The medication suppresses a protein that people with congenital anodontia have, and that is what makes them start growing teeth. Normal people do not have that protein issue, so the drug can’t help.
Given the average quality of science reporting and the lack of actual journal articles, it might be the same thing with a misunderstanding in either the earlier article or this one.
science reporting is awesome, I love when people start pushing random BS because they decided they needed to “dumb it down” because they themselves were too dumb to get it in the first place
Well that’s what happened with this article. Their little mention of growing teeth lost from cavities is complete hyperbole. This treatment suppresses a protein found in the less than 1% of people who have congenital anodontia. It can’t be used at all to help grow teeth for anyone else.
Oh man. It seems the only misunderstanding is that no one else in here understands the article or what congenital anodontia actually is. So what I had initially stated, and per this article, it won’t help 99% of people.
How do we not understand the article? If the article is full of bull, that’s another story
The context and explanation of everything else in the article in everything but that one cavity paragraph explains what it can treat and how it does it. Then that one paragraph says it may be used. In full context of the entire article (which is trash) it can be interpreted as not really for cavity missing teeth.