The Food and Drug Administration announced Friday it had broadened the approval of the FluMist nasal spray to become the first “self-administered” influenza vaccine — though a delay in the change means the vaccine will not be available to ship to homes until next year’s flu season at the earliest.
“Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility and accessibility,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
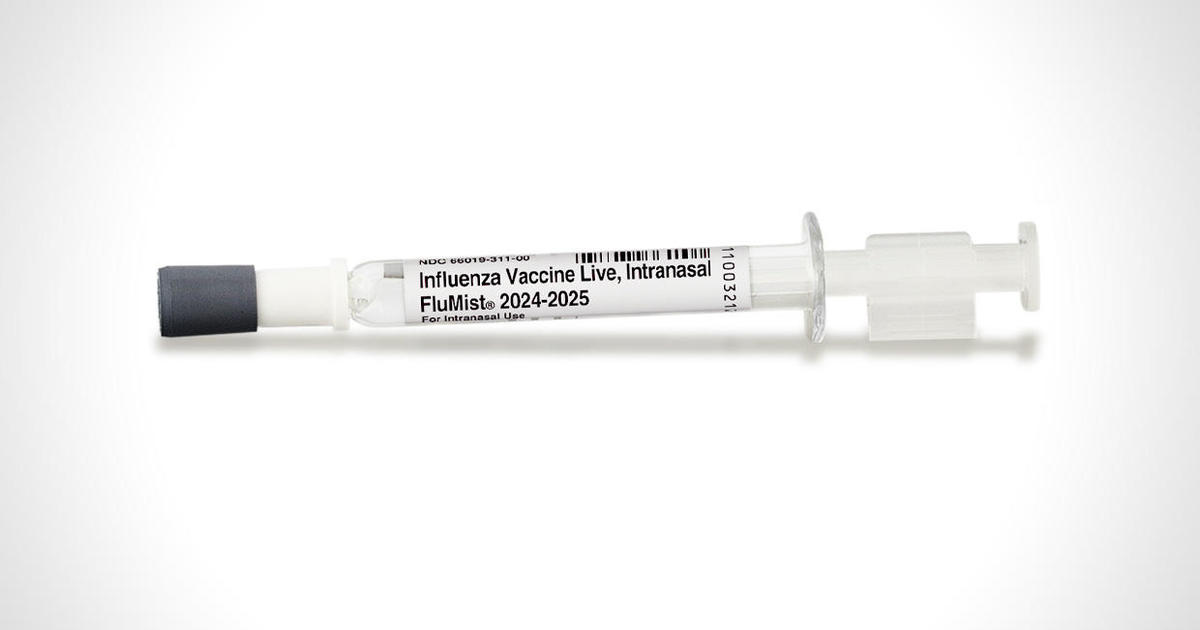
The FluMist vaccine, manufactured by AstraZeneca, had previously been approved back in 2003 to be given by health care providers similar to other flu shots. Now the vaccinemaker has approval to sell FluMist to adults for use at home on themselves or to administer to their children.


Assuming the unit-price is comparable, that’s going to save a hell of a lot of money.
I just went to the drive-through (spot the American) COVID/Flu shot clinic. There were probably 40-50 people working there, directing traffic and jabbing arms all day.
Assuming it works, I wouldn’t be surprised if they phase out the injection in a few years.
They’ve had inhalable flu vaccines for a bit though limited in terms of availability. The shot is a fair bit cheaper though so be interesting to see whether this is cheap enough in the current antiscience environment to stick (haha) around. Even if this works for flu we are still going to be getting covid boosters via a needle for a number of years at least.