Wish they gave the current energy density of lithium batteries for comparison.
Here’s a neat comparison of how different chemistries preform. Higher up is higher energy per weight.
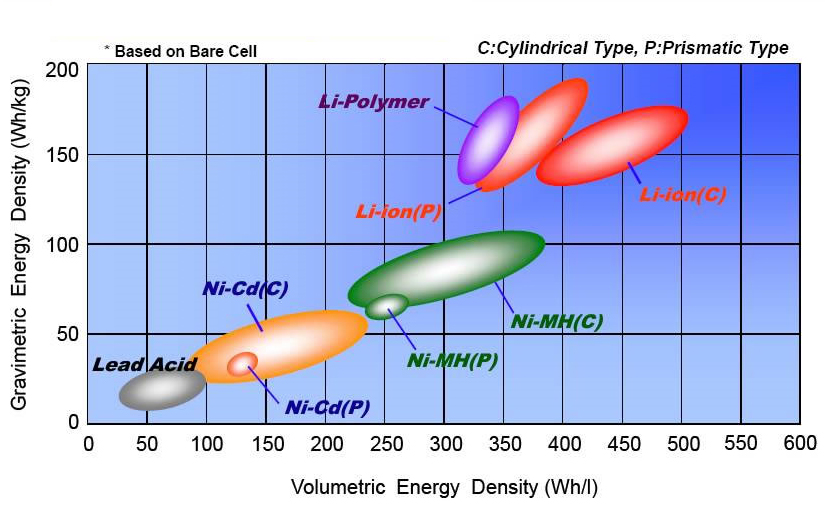
Looks like this is pretty old though
It’s a range, but LFP is generally in the 90-160wh/kg and NMC (lithium manganese cobalt oxide) tops out around 275 wh/kg.
It’s not quite double, but doubles the density of most mass manufactured cells. Time will tell if it gets to scale, but it’s a step in the right direction.
Feeling stupid: doesn’t this mean more energy per kilogram is possible with Na cells?
deleted by creator
Lithium ion is around 265-280wh/kg but hydrogen is already in the kWh
Isn’t most of the weight with hydrogen coming from the high pressure tank and gas regulator?
My impression is that the gas is light but whole system hydrogen is pretty heavy.
edit: Did some more reading. Hydrogen is still competitive on a Wh/kg basis, but worse on a Wh/L basis. Larger tanks are harder to fit in passenger cars than batteries and hydrogen’s poor whole system efficiency has kept fuel prices high while lithium batteries and solar power keep getting cheaper.
H2 to get weight of tank down and highest density can be liquified. This works best by far for aviation that refuels right before takeoff. Can work for commercial boats as well. Costs more energy to liquify than compress.
This makes hydrogen even more expensive and pushes it further into niches which need maximum range at any cost.
Renewable h2 can be cheaper than gasoline or kerosene. Even with liquifaction. Has to use behind the meter or wholesale renewables instead of fixed utility pricing with transmission costs.
Planes typically spend 100x in fuel over lifetime compared to price of plane
Where are you finding this cheap renewable H2?
Or is this a theoretical future development?
Your next spicy pillow may be a salty pillow
I work in the electronics industry and had never heard the term “spicy pillow”. I’ll hand in my badge.
https://www.powerbankexpert.com/lithium-battery-spicy-pillow/
There’s a Lemmy community for pictures of spicy pillows. It is good.
Good to hear. Let’s hope it actually works at scale.
We’re increasingly eating so much salt that at some point maybe The Matrix plot will end up making sense.
I wonder what the machines will use all the microplastics for.
Nanomachines, son!
Here’s the original press report https://uh.edu/news-events/stories/2024/december/12202024-canepa-batteries.php
Vanadium, including flow batteries, have never provided useful energy storage solutions, and never will.
The flow application is a scam for vanadium producers to take supply off the market for an already extremely expensive commodity. The advantage is that it does not need recycling to sell it off.
Consuming it in batteries would be ultra expensive batteries. Lithium much more abundant, as is nickel and cobalt if needed
Sodium batteries are thermal batteries, right? So they need turbines to recover the energy into electricity? Or are these chemical sodium batteries?
Chemical. It’s in the article:
Battery prototype
The researchers also created a battery prototype using the new material, NaxV2(PO4)3, demonstrating significant energy storage improvements. NaxV2(PO4)3, part of a group called “Na superionic conductors” or NaSICONs, is designed to let sodium ions move smoothly in and out of the battery during charging and discharging, according to a press release.
The material has a unique way of handling sodium, allowing it to work as a single-phase system. This means it remains stable as it releases or takes in sodium ions. This allows the NaSICON to remain stable during charging and discharging while delivering a continuous voltage of 3.7 volts versus sodium metal, higher than the 3.37 volts in existing materials, according to researchers.
I wonder, though, if having to use Vanadium defeats the point of dropping Lithium for Sodium.
Sodium batteries are thermal batteries, right? So they need turbines to recover the energy into electricity?
Are you thinking of concentrated solar, maybe?
I think I was mixing those up with thermal energy storage and thermal batteries.
https://en.m.wikipedia.org/wiki/Thermal_energy_storage
https://en.m.wikipedia.org/wiki/Molten-salt_battery#Thermal_batteries_(non-rechargeable)
Sodium batteries do have a high temperature type, but it does look like they are non rechargeable and do generate electricity directly. The thermal energy storage only stores thermal energy rather than electricity, but they use sand.







